20 Nov, 2025
The solution to the carbon problem could be carbon itself
Can we use carbon to help decarbonise the world and transform the energy and chemical industries?
Yes, it seems, but there are some key challenges to overcome first.
A review article in the latest Science Advances pinpoints the potential of transforming carbon emissions into innovative catalysts used in electro- and photocatalysis systems to facilitate cost-effective decarbonisation and avoid the need to use critical minerals that come with high costs and geopolitical risk.
With the energy intensive nature and large contribution to greenhouse gas emissions of many chemical processes, researchers have put considerable effort into finding ways to convert CO2 into non-volatile carbon products and storing them, which alone, even if successful, will not offset carbon at a large scale.
Carbon, as a product used in research and industry, can also be synthesised through techniques such as chemical vapour deposition, typically using hydrocarbons as the source.
The review authors from the ARC Centre of Excellence for Carbon Science and Innovation (COECSI) argue that a more practical approach is to convert CO2 into high-value catalysts that can then be used to convert CO2 or carbon wastes into fuels and chemicals.
“Achieving this will advance climate goals, material innovation and contribute to a circular carbon economy,” says COECSI Director and corresponding author, Prof Liming Dai.
What makes carbon a good catalyst
Carbon is strong, conductive and stable in the harsh conditions found in electrocatalytic cells, but carbon is catalytically inactive.
Prof Dai led a team that discovered a way to transform carbon into efficient catalysts for energy conversion. Catalytic active centres (CACs), where reactions occur, can be formed in carbon with some clever engineering at the atomic scale that includes methods such as heteroatom doping, molecular adsorption and defect engineering to break the electron symmetry in carbon’s aromatic rings.
A promising area of research is the concept of doping to introduce multifunctional properties to CACs where heteroatoms and carbon atoms work in synergy to markedly enhance catalytic performance. For example, researchers developed N and P co-doped bifunctional porous graphene catalysts for Zn-air batteries that have near theoretical energy capacities.
The efficacy of the CAC is further influenced by the architecture of the carbon substrate. For example, a novel 3D tunable carbon nanotube-graphene pillar structure that resembles a field of city skyscrapers has introduced marked improvements in ion and electron transfer and a greater number of accessible CACs.
CO2 as a carbon source
Different processes have been employed, each with advantages and limitations, to catalytically convert CO2 into a range of carbon-based nanostructures such as graphene carbon nanoparticles, nano-onions, nano-platelets and carbon nanotube wool.
One straight forward method that thermally reduced CO2 with magnesium produced hollow-structured mesoporous carbon cubes that have shown promise as an alternative to commercial platinum-carbon catalysts in fuel cells.
A COECSI team has also converted CO2 into edge-doped graphene electrocatalysts by co-ball milling graphite with dry ice.
Typically, electrocatalytic conversion of CO2 to carbon is an energy intensive process because of the need for high temperatures.
While still at the fundamental stage, a recent promising cerium-containing liquid metal (gallium) electrocatalytic converter successfully converted CO2 into carbonaceous and graphitic products at ambient temperature.
The economics and pursuit of multi-carbon products
While the review authors note limitations with each method to transform CO2, they also note the techno-economic potential for cost-effective synthesis of carbon materials from CO2.
“As the market price for carbon nanotubes (CNTs) currently exceeds $100,000 per ton, we have identified economically attractive methods to convert CO2 into carbon catalysts as alternatives to expensive noble-metal catalysts,” says lead author, Prof Zhenhai Xia.
“The economics of these technologies start making sense when you also consider that to build a circular economy, we will be abating the carbon. That is, previously seen as waste, carbon now has a market value and there is an environmental penalty and cost associated with not abating carbon waste such as CO2,” says Prof Rose Amal.
Further value will be realized with the economic production of comparatively high value multi-carbon products such as synthetic fuels and industrial chemical such as ethylene.
“The challenge remains, however, to boost catalytic efficiency and yields,” says Prof Shizhang Qiao
One promising method may come in the form of metal-free and metal-doped carbon catalysts. For example, a metal-free carbon catalyst using N-doped graphene quantum dots converted CO2 to higher order hydrocarbons such as ethylene and ethanol.
Carbon catalysts have also been used to convert nitrogen and nitrite wastes to ammonia and urea, essential for fertilisers and pharmaceuticals.
From a systems perspective, there remains also the challenge of scalability and price. Further, integrating CO₂ capture, conversion, and material synthesis into a cost-effective, sustainable system remains technically complex.
Despite these challenges, CO₂-derived carbon materials offer distinct advantages over those produced from fossil-based feedstocks. They provide a sustainable and renewable carbon source, help close the carbon loop, and potentially reduce production costs when using waste CO₂ streams,” says Prof Dai.
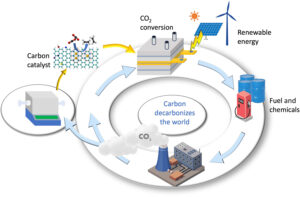
Transforming carbon emissions into carbon catalysts for sustainable energy and green chemistry—a novel strategy to use carbon in decarbonizing the world.
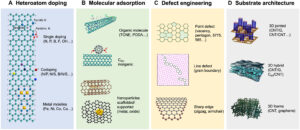
(A) Heteroatom doping. (B) Molecular adsorption. TCNE, tetracyanoethylene. (C) Defect engineering. (Middle) Reproduced from (132). Copyright 2019, American Chemical Society. (Lower) Reproduced from (133). Copyright 2022, Elsevier. (D) Substrate architecture. (Middle) Reproduced from (134). Copyright 2015 RSC Publishing. (Lower) Reproduced from (7). Copyright 2015 Springer Nature.
Full citation: Zhenhai Xia et al., Carbon catalysts for CO2 conversion: From carbon emissions to zero-carbon solutions. Sci. Adv.11, eady9164(2025). DOI:10.1126/sciadv.ady9164